- Home
- betapharm-co
- LYDASE
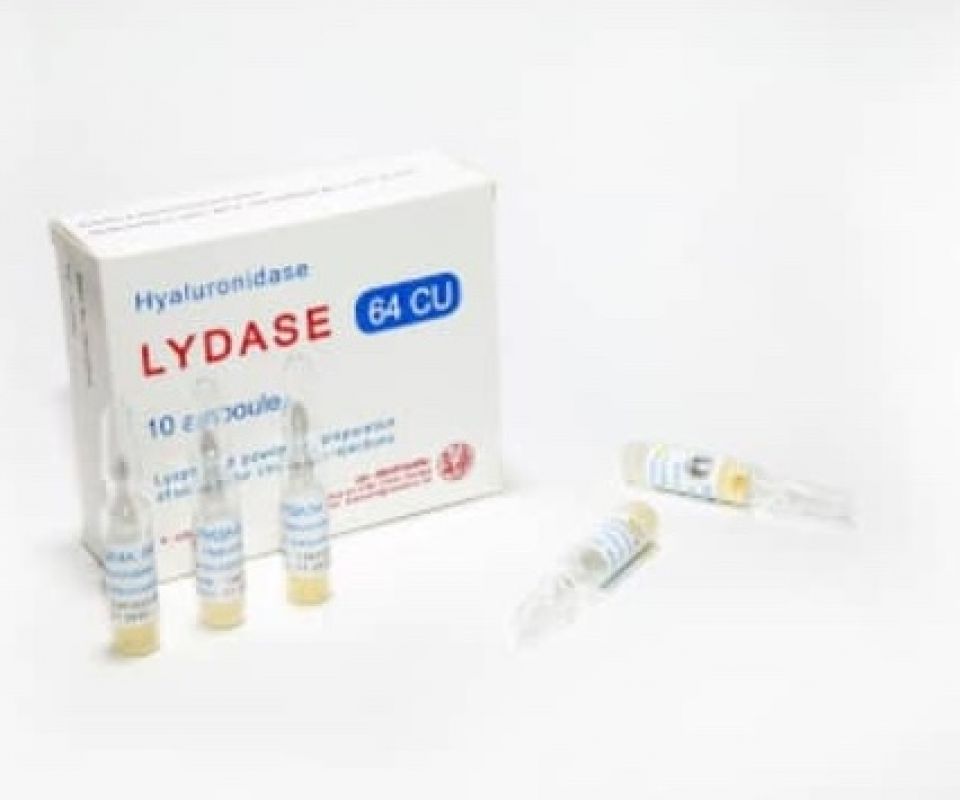
LYDASE
An enzyme product isolated from spermarium of heavy beasts. It breaks down the main component of the ground substance of the connective tissue
Trade name of the drug: Lydazum 64УЕ.
Generic name (INN): Hyaluronidase
Pharmaceutical form: Dried cake for preparation of a solution for injection and local administration.
Formulation: 1 ampoule contains: Active moiety: Hyaluronidase from spermarium of heavy beasts 64 oz.
Description: Lyophilized porous mass in the form of a beige colored tablet of different intensities.
Pharmacological group: enzyme product
ATC–code: B06AA03
Pharmacological effect: An enzyme product isolated from spermarium of heavy beasts. It breaks down the main component of the ground substance of the connective tissue – hyaluronic acid (mucopolysaccharide, which contains acetylglucosamine and glucuronic acid, is a cement substance of the connective tissue), reduces its viscosity, increases tissue and vascular permeability, facilitates the movement of fluids in intertissue spaces; reduces swelling of the tissue, softens and flattenes scars, increases the range of motion in the joints, reduces contractures and prevents their formation. Hyaluronidase causes the breakdown of hyaluronic acid to glucosamine and glucuronic acid and thereby reduces its viscosity. The duration of action with subcutaneous administration is up to 48 hours.
Indications for use: Keloids, traumatic, postoperative scars; long non-healing ulcers (including radiation) Dupuytren’s contracture; joint stiffness, joint contracture, (after inflammatory processes, injuries), osteoporosis, ankylosing spondylitis, severe diseases of the lumbar disc; chronic tendovaginitis, scleroderma (skin manifestations), hematoma of soft tissues of superficial localization; preparation for skin plastic surgery for cicatricial contraction. Pulmonary tuberculosis (complicated by nonspecific lesions of the bronchi), inflammatory processes in the upper respiratory tract and bronchi with obstruction. Traumatic lesions of the nerve plexuses and peripheral nerves (plexitis, neuritis). Hyphema, hemophthalmus, retinopathy of various etiologies.
Dosage and Administration: In case of cicatricial lesions subcutaneously (under the scar-altered tissue) or intramuscularly (near the lesion site), 64 oz (1 ml) are administered daily or every other day (a total of 10-20 injections).
In case of traumatic lesions of the nerve plexuses and peripheral nerves, they are injected subcutaneously into the area of the affected nerve (64 oz in procaine solution) every other day; 12-15 injections per course. The course of treatment is repeated if necessary.
When administered in ophthalmic practice, the drug is administered subconjunctivally – 0.3 ml, parabulbarly – 0.5 ml, as well as by electrophoresis.
When applied by electrophoresis, 64 oz is dissolved in 60 ml of distilled water, 2-3 drops of a 0.1% hydrochloric acid solution are added and injected from the anode into the affected area for 20-30 minutes. The course of treatment is 15-20 sessions. The prepared solution should be used within 24 hours.
Contraindications: Hypersensitivity, acute infectious-inflammatory diseases, recent hemorrhages, acute intercurrent diseases, children under 18 years of age. For inhaled administration – pulmonary tuberculosis with severe respiratory failure; pulmonary, bleeding, hemoptysis; malignant neoplasms, recent vitreous hemorrhage. Concomitant administration of estrogen. Data on the administration of the drug in children are not available.
Side effects: Allergic reactions; with prolonged use – a local irritative effect.
Pregnancy and lactation: During pregnancy and lactation, the drug is used with caution, strictly as prescribed by the doctor!
Drug interactions: It improves the absorption of drugs administered subcutaneously or intramuscularly, enhances the effect of local anesthetics.
Special warnings: The solution should not be administered through a catheter into which solutions containing cations have previously been injected.
Solutions for injection are prepared in 0.9% sodium chloride solution or 0.5% procaine solution, for electrophoresis – in distilled water.
It should not be administered into zones of infectious inflammation and tumors.
In acute hemorrhages, Lydazum should not be administered.
In ophthalmology, parenteral administration only after checking for the lack of individual sensitivity to the drug is recommended.
Overdose: Symptoms: rigor, nausea, vomiting, dizziness, tachycardia, decreased arterial blood pressure, local edema, urticaria, erythema.
Treatment: administration of epinephrine, glucocorticosteroids; antihistamines.
Drug form: Lyophilisate for preparation of a solution for injection. 64 oz in glass ampoules with a nominal volume of 2 ml, 5 ampoules in PVC pallets, 2 pallets with instructions for use, in a printed cardboard box.
Shelf life: 2 years.
Storage conditions: In a place inaccessible to children, protected from light at a temperature from + 20C to + 80C.
Dispensing rules
By prescription.