- Home
- betapharm-co
- DESMOPRESSIN
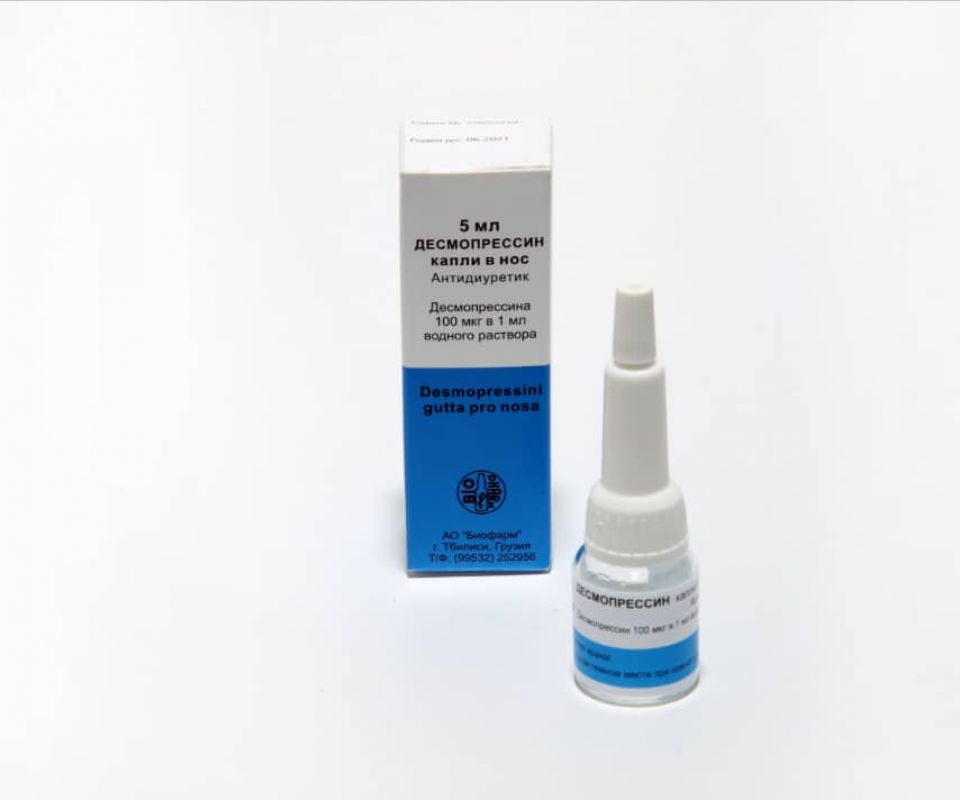
DESMOPRESSIN
The drug contains desmopressin – a synthetic analogue of the posterior pituitary natural hormones – arginine-vasopressine (antidiuretic hormone).
Trade name of the drug: Desmopressin
Active agents (INN): Desmopressin (desmopressinum)
Pharmaceutical form: Drops
Formulation:
Each ml of the drug solution contains:
Active moiety: desmopressin – 100 mcg;
Excipients: benzalkonium chloride, sodium chloride, sodium phosphate monosubstituted dihydrate, purified water.
Description: transparent colorless liquid.
Pharmacological group: antidiuretic (hormonal drug).
Pharmacological properties
The drug contains desmopressin – a synthetic analogue of the posterior pituitary natural hormones – arginine-vasopressine (antidiuretic hormone). Compared with vasopressine, desmopressin has a less pronounced effect on the unstriated muscles of blood vessels and internal organs with a more pronounced antidiuretic activity, which is due to changes in the structure of the desmopressin molecule compared to the natural vasopressin molecule: deamination of 1-cysteine and replacement of 8-L-arginine with 8 D-arginine.
After entering the body, the drug is rapidly absorbed and vasopressin acetate enters the bloodstream. The antidiuretic effect occurs within 30 minutes after taking the drug. The intake of 10-20 micrograms of desmopressin intranasally provides in most patients an antidiuretic effect lasting 8-12 hours.
The toxicity of the drug is very low. No teratogenic or mutagenic effects of desmopressin have been identified.
Desmopressin begins to be detected in the blood 15-30 minutes after administration. The bioavailability of the drug after its intranasal administration is 3-6%. The maximum plasma concentration is reached after 60 minutes. The biotransformation of desmopressin is carried out in the liver and consists in the degradation of the disulfide bond by the transhydrogenase enzyme. Desmopressin is destroyed more slowly than the natural antidiuretic hormone. Desmopressin is excreted mainly by the kidneys.
The elimination half-life of desmopressin is 3-4 hours.
Indications for use
– diabetes insipidus of central origin (diabetes insipidus);
– primary nocturnal enuresis in patients older than 5 years with normal concentration ability of the kidneys;
– acute polyuria of central genesis (a consequence of trauma, disease or surgery on the central nervous system);
– test for determining the concentration ability of the kidneys
Dosage and Administration
The drug should be instilled with a slight throwing of the patient’s head back and to the side directly into the nasal passages towards the nasal septum. The number of drops is measured using a pipette, which is part of the cap of the bottle with the drug. The therapeutic effect of the drug begins to appear within 30 minutes after administration.
Diabetes insipidus (diabetes insipidus). The use of desmopressin for the treatment of patients with an established diagnosis leads to a significant decrease in the volume of urination, which is manifested in a decrease in the frequency of desire to urinate. The dose and intervals between administration of the drug are determined by the attending physician individually for each patient, depending on the sensitivity to the drug. In addition, it should be borne in mind that only a small proportion of patients have complete blockade of the secretion of antidiuretic hormone.
The daily dose for children and adults is from 5 to 40 micrograms of desmopressin, i.e. from 1 to 8 drops and can be divided into 3 doses.
Initial nocturnal enuresis (enuresis nocturna). An effective dose is selected individually and usually it is 10-30 micrograms of desmopressin per day. A suitable dose is determined during treatment by gradually increasing it. Therapy begins with the lowest dose – 1-2 drops for children with a body surface area not exceeding 1 sq.m. or with 2 drops for children with a body surface area exceeding 1 sq.m. If there is no effect from the administration of the drug for 2-4 weeks, the daily dose is increased by 1 drop. The process of dose selection (up to 30 – 40 mcg per day) continues until the effect of therapy. During the course of treatment with the drug, fluid intake should be limited at night. In case of signs of fluid retention in the body – treatment should be stopped immediately.
Determination of the concentrating ability of the kidneys. The recommended dose is 3 drops (about 15 mcg of desmopressin), and for children over 1 year old – from 2 to 3 drops (about 10-15 mcg of desmopressin). After taking the drug, the patient should empty the bladder. Over the next 4 hours after administration the drug, urine samples of the patient’s urine are collected every hour to determine osmolarity. During the test time and 4 hours after its completion (in total within 8 hours after the administration of the drug), it is necessary to limit the intake of fluid.
For children under 1 year of age, a study of the concentrating ability of the kidneys with the drug is carried out only in exceptional cases, because at this age the concentrating ability of the kidneys is reduced in comparison to that in older children. A study of the concentrating ability of the kidneys should be performed by a pediatric nephrologist. The administration of too large doses in children aged about 1 year may be accompanied by the development of spasmus caused by irritation of the nervous system. In the case of a test for children aged 1 year, during the collection of urine should completely exclude fluid intake.
If the test found a decrease in the concentrating ability of the kidneys – the test should be repeated.
The acute form of central polyuria. The drug is administered in 2 drops. Fluid intake and diuresis are evaluated at hourly intervals until full balance is obtained, and the incipience of desmopressin effect can be expected within 30 minutes after drug administration. Over the next 3-5 hours, the correspondence of the fluid received and released to the body is assessed, the plasma and urine osmolarity is monitored, and the sodium concentration in the blood is monitored. In cases where the state of acute central polyuria lasts more than 1 hour, parenteral (usually intravenous) administration of the drug is recommended.
Administer strictly as directed by the doctor!
Side effects
Weakly expressed, rare and disappear with a decrease in dose. More common than others: headache, nausea, pain in the stomach, coryza, nosebleeds. Less commonly – skin manifestations of allergic reactions to the components of the drug.
Treatment with the drug without limiting fluid intake can cause water retention in the body, which is manifested by an increase in body weight, edema, and a decrease in the level of sodium in blood plasma.
Contraindications
– all conditions accompanied by reduced plasma osmolarity, including psychogenic or habitual polydipsia;
– decompensated heart failure;
– fluid retention in a body;
– Anuria and other conditions requiring the use of diuretics;
– Hypersensitivity to the components of the drug.
Drug interactions
The administration of desmopressin while taking indomethacin, which causes an increase in the sensitivity of the kidney receptors to the antidiuretic hormone, is accompanied by a more pronounced effect.
In cases where the hypophysary function is partially or fully preserved, the effect of desmopressin can be enhanced while prescribing drugs that stimulate the secretion of its own antidiuretic hormone – tricyclic antidepressants, chlorpropamide, carbamazepine and clofibrate.
Special warnings
Particular caution when using desmopressin should be observed in cases:
– treatment of children under the age of 1 year and elderly patients;
– the presence of water-electrolyte imbalance in the patient’s body;
– the potential danger of increased intracranial pressure.
In the treatment of nocturnal enuresis and in the determination of the concentrating ability of the kidneys, the patient should not take any liquid 1 hour before – and within 8 hours – after administering the drug. In case of thirst, it is allowed to take no more than 200 ml during the entire time period indicated above. If you need to increase fluid intake (for example, in case of fever), discontinue therapy and consult your doctor. Fluid intake during the day should not limited or normal fluid intake should not disrupted.
During the treatment of nocturnal enuresis, it is recommended to control the osmolarity of urine. If necessary, plasma osmolarity should be determined.
Since the drug is used as drops in the nose, all changes in the nasal mucosa, including acute and chronic inflammation, can cause fluctuations in the amount of the drug entering the bloodstream. In such cases, the number of entered drops should be changed.
Pregnancy and lactation
The administration of the drug is not contraindicated during pregnancy and lactation.
Impact on the ability to drive a car and manage complex mechanisms
Taking the drug does not adversely affect the ability to drive a car.
The drug should not be used after the expiration date and should be stored out of the reach of children.
Overdose
It leads to an increased risk of fluid retention in the body and hyponatremia. Despite the fact that the treatment of hyponatremia is strictly individual, in the absence of symptoms, it is recommended to discontinue treatment with desmopressin and limit fluid intake.
Patients with a high degree of fluid retention are prescribed isotonic or hypertonic sodium chloride solution. If necessary, furosemide may be prescribed.
Drug form
5 ml of 100 μg / ml solution of desmopressin (drops in the nose) in glass vials. In the package there is 1 vial equipped with a pipetting head.
5 ml of solution in the form of drops in the nose in a vial with a pipette. 1 ml of the solution contains about 20 drops. 1 drop of solution contains about 5 μg of active moiety. In the vial only about 100 drops.
Storage conditions
Store in a dry, dark place protected from children, at room temperature.
Shelf life
2 years
Dispensing rules
By prescription.