- Home
- betapharm-co
- BETRIVIT
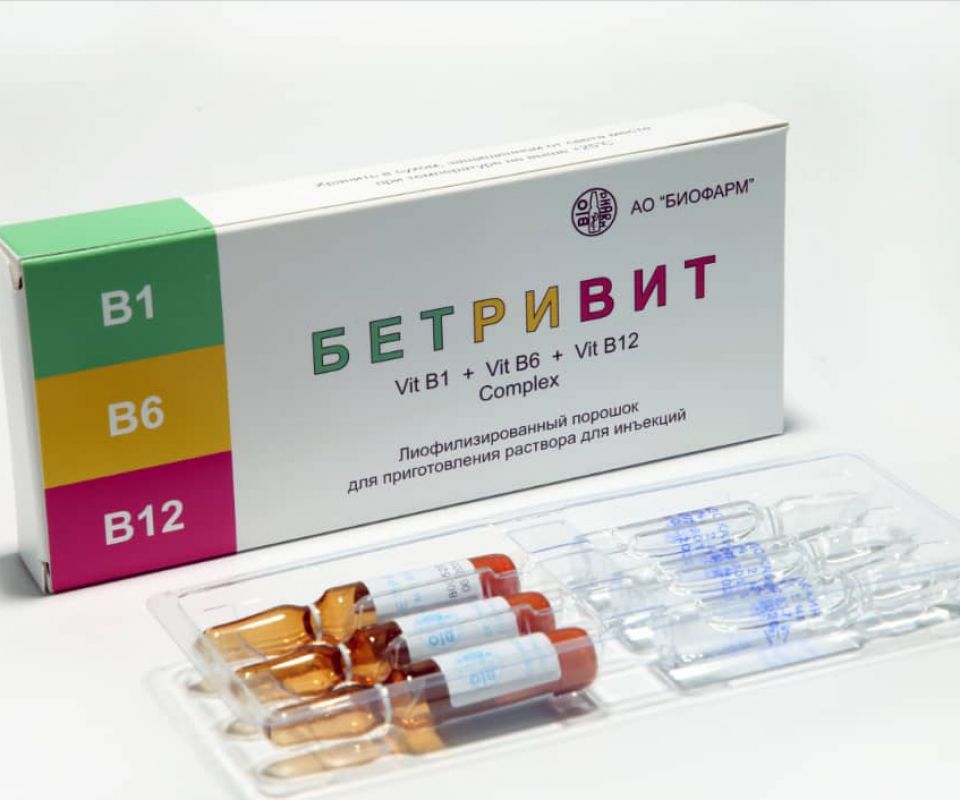
BETRIVIT
The drug is a complex of neurotropic vitamins (B vitamins).
Pharmacotherapeutic group: Complex vitamin preparation
INN: Vitamin B1 + Vitamin B6 + Vitamin B12 complex.
Drug form: Lyophilized powder of the drug in an ampoule in set with a solvent ampoule for the preparation of a solution for injection.
Formulation: Each ampoule of the drug contains
Active moiety: Thiamine chloride (Vitamin B1) – 50 mg, pyridoxin g / x (Vitamin B6) – 50 mg, cyanocobalamin ((Vitamin B12) – 0.5 mg.
Excipients: Methylparaben.
Each ampoule of solvent contains: 2 ml of a 0.5% solution of lidocain g / x.
Pharmacological properties:
Pharmacodynamics: The drug is a complex of neurotropic vitamins (B vitamins). It has a therapeutic effect on inflammatory and degenerative processes occurring in the muscles of the musculoskeletal system. It is used to compensate for vitamin-deficient conditions, in high doses it has an analgesic effect. Strengthens blood circulation, regulates the action of the nervous system and the process of blood formation.
Pharmacokinetics: The active components of the drug are mainly accumulated in the liver, where they turn into physiological metabolites. It is excreted from the body with urine (Vit. B1 and Vit. B6) and with bile (Vit. B12).
Indications for use: Diseases of the nervous system of various etiologies, incl. neuritis; neuralgia; neuropathies and angiopathies; Diabetic alcoholic and other myodynia; retrobulbar neuritis; facial paresis. Also, at the time of a confirmed neuralgic diseases caused by deficiencies of Vitamins B1 and B6.
Dosage and administration: Injection deep into the muscle! For effective absorption, massage at the injection site within 1-2 minutes is recommended. 1 ampoule of the drug daily or 2 ampoules every other day. The duration of the course is 6 days. An additional course as prescribed by a doctor.
Contraindications: Acute and severe forms of decompensated heart failure. Individual sensitivity to the components of the drug.
Side effects: In rare cases, local allergic reactions associated with individual hypersensitivity to the components of the drug are possible.
Pregnancy and lactation: During pregnancy and lactation, the use of the drug is not recommended.
Driving or working with other mechanisms: No restrictions.
Interaction with other drugs: The drug (pyridoxine) reduces the therapeutic efficacy of levodopa. It is unacceptable to mix the drug in one syringe with other drugs.
Overdose: There is no evidence of an overdose.
Special warnings: Apply the solution of the drug immediately after its preparation! Do not use the drug solution if the color has changed (the color of the freshly prepared solution should be bright red). In case of serious anaphylactic reactions, 1-5 ml of 0.01% adrenaline solution and up to 10 mg of dexamethasone are intravenously administered.
Packing: 3 ampoules of the preparation and 3 ampoules of the solvent in a PVC tray with instructions for use in cardboard pack.
Storage conditions: In a dry, dark place at a temperature of no more than + 250C.
Shelf life: 2 years.
Dispensing rules: By prescription!